The last couple of months has seen a glut of exciting new papers published by Lister Fellows. In this article, we explore papers published by Dr Erica Watson of the University of Cambridge, Dr Sebastian Guettler of the Institute of Cancer Research, and Professor Sherif El-Khamisy of the University of Sheffield.
Dr Erica Watson’s recent paper in Nature Communications identified that folic acid deficiency alters the sperm epigenome and is associated with transcriptional memory in grandchildren.

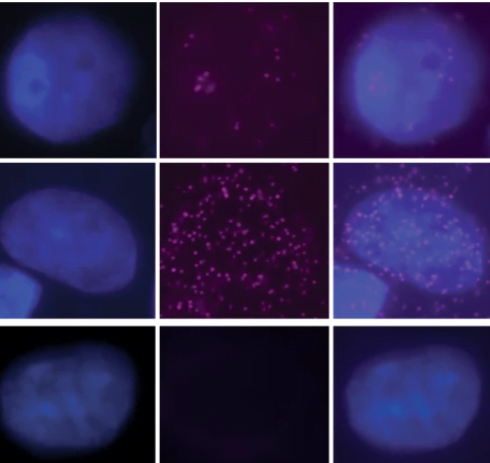
The paper, “Defective folate metabolism causes germline epigenetic instability and distinguishes Hira as a phenotype inheritance biomarker”, explores how diseases are inherited due to environmental stressors rather than genetic mutations.
Folic acid is known to cause spina bifida and other birth defects. The current study follows on from Erica’s previous work that found that a mutation in the Mtrr gene causes metabolic changes in mice similar to folic acid deficiency in humans. This mutation also has detrimental effects on the development of the great grandprogeny even though they do not carry the Mtrr mutation.
The new paper provides evidence that the Mtrr mutation substantially changes the sperm’s epigenome, which is likely to be the mechanism for the inheritance of development defects. The lab’s research found that the mutation leads to changes in methylation of sperm DNA, and in some cases these changes are found in known regions of the genome that are protected from the normal ‘generational wiping’.
Genes associated with methylation changes in these regions were misexpressed, which was associated with increased risk of detrimental effects on health. One of these genes was Hira. Since HIRA protein is part of the cell machinery that regulates the epigenome in sperm and eggs, its misexpression represents a candidate biomarker of so-called epigenetic inheritance, and perhaps it is even a molecular mediator of this type of non-genetic inheritance.
It may be that the grandprogeny ‘remembers’ the epigenetic changes caused by folic acid deficiency in their grandfather’s sperm, which impacts gene expression and disease risk.
Overall, this research solidifies, at least in mice, that the detrimental effects of folic acid deficiency on health can last for multiple generations and moves us closer to understanding the molecular mechanism behind this phenomenon.
At the end of July, Erica also published the paper “Variably methylated retrotransposons are refractory to a range of environmental perturbations” in Nature Genetics. This work was done in collaboration with the Ferguson-Smith and Ozanne labs at the University of Cambridge and with labs at the University of Philadelphia.
This work revealed that some retrotransposons are not as sensitive to environmental factors – and more resistant to the effects of ageing – as previously thought. This fascinating research challenges the belief that the variably methylated intracisternal A-particle (VM-IAP) group of transposons are sensitive to changes in the environment.
The study explored the effects of maternal exposure to the endocrine disruptor bisphenol A, an obesogenic diet or methyl donor supplementation on mouse VM-IAPs. It also looked at a mouse model that simulates folic acid deficiency. This revealed changes in VM-IAP methylation levels and gene expression, but the researchers conclude that this is driven by transcription factors and more complex interactions between the genome and epigenome.
You can read more about Erica’s research here.
Published in September in the prestigious Nature Communications, Lister Fellow Sherif El-Khamisy’s latest paper identifies a DNA switch that plays a vital role in regulating the genome.

Further research into this genetic pathway could eventually lead to new treatments for diseases such as cancer, dementia, and conditions which affect coordination, such as Huntington’s Disease.
The full paper, “USP11 controls R-loops by regulating senataxin proteostasis”, is available to read now.
The study is the first to explain how the number of R-loops in DNA are regulated, which form during DNA metabolism and help regulate protein production. Three proteins – USP11, KEAP1, and SETX – work together to prevent overproduction of R-loops, which can lead to DNA instability and cell death, and to ensure optimal levels for the manufacture of the proteins that the body needs.
There is potential that finding ways of controlling the behaviour of this protein trio could help detect and treat serious diseases caused by DNA mutations. Drugs to control the modification of one or more of these proteins could be used to kill cancer cells or reduce genome damage to address ageing and neurodegeneration. The team aims to start that drug development process next year.
Sherif is the Co-Founder and Deputy Director of the Healthy Lifespan Institute at the University of Sheffield.
Find out more about his research here.
Dr Sebastian Guettler’s recent paper reports on his lab’s latest findings about the precise mechanisms of the Wnt/β-catenin pathway, which is commonly mutated in cancer.

Sebastian’s research used a unique approach to find out how the multi-protein β-catenin destruction complex kickstarts β-catenin degradation.
“Our approach enables us to interrogate the inner workings of the destruction complex at great detail and investigate the precise impact of cancer mutations in one of the destruction complex components, which occur in up to 80% of colorectal cancers,” says Sebastian.
The study revealed that AXIN1 polymerization and APC promote β-catenin capture, phosphorylation, and ubiquitylation.
The key part of this research was developing a suitable in vitro system for studying the molecular mechanisms involved. The system may prove a useful tool in identifying and developing molecular probes and therapies for controlling this pathway in cancer.
The open access paper, “Reconstitution of the destruction complex defines roles of AXIN polymers and APC in β-catenin capture, phosphorylation, and ubiquitylation”, was published in August in Molecular Cell.
Sebastian is Deputy Head of the Division of Structural Biology at the Institute of Cancer Research. He won the Lister Prize in 2017.
You can read more about the Guettler Lab’s research on their website.



