We are pleased to announce that one of our valued Lister Institute Research Prize Fellows David Komander has co-authored two articles in the prestigious journal Nature recently.
David is group leader at the Medical Research Council’s Laboratory of Molecular Biology where he studies specificity in the ubiquitin system.
Ubiquitination is the act of adding ubiquitin (a regulatory protein found in most eukaryotic tissue) to a protein substrate, and it has been found to play an important role in regulating a number of physiological processes at the cellular level.
For example, ubiquitination is responsible for the selective degradation of several short-lived proteins in eukaryotic cells (organisms which contain genetic material, nuclei and other organelles bound by membrane). The deregulation of ubiquitination has been linked to diseases in humans including both cancer and neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease. Research is therefore underway to better understand how ubiquitin effects and disrupts cellular processes.
David’s first co-authored paper in Nature is entitled Molecular basis of USP7 inhibition by selective small-molecule inhibitors, and it discusses new findings on how an enzyme called ubiquitin-specific protease 7 (USP7) can be inhibited specifically by drug-like small molecules, which in turn leads to the re-activation of the tumour suppressor p53.
P53 is the name given to a set of proteins with an important role in combating many cancers. Its ability to limit or prevent mutation of human genomes has even led to it being dubbed “the guardian of the genome” (source). In over half of human cancers p53 is lost or mutated in multiple ways, and therefore approaches to stabilising or re-activating it are a key goal in current pharmaceutical research.
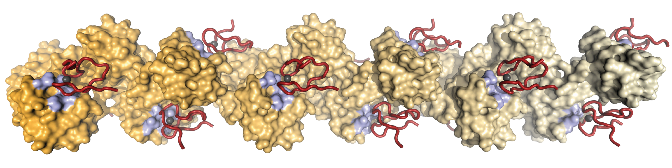
The paper co-authored by David, as well as several other papers published simultaneously in Nature and elsewhere, demonstrates some real progress in this area. A collaborative team, the DUB Alliance that includes David and scientists in his lab, were able to identify two specific USP7 inhibitors (FT671 and FT827) which cause the re-activation of p53 and, potentially, the subsequent suppression of tumours. These small molecules work by removing cellular breaks, in this case the oncogene MDM2, from cells, by increasing their ubiquitination and hence destruction. It is hoped that the findings will help inform important pharmaceutical research in this emerging area of drug discovery.
In the second paper in Nature, David and his team revealed the three-dimensional structure of an enzyme modifying ubiquitin with phosphate groups, a kinase termed PINK1. Mutation of this protein in humans causes inherited, early-onset forms of Parkinson’s disease, a highly devastating neurodegenerative disorder.
The first structures of PINK1 bound to ubiquitin explained molecularly how mutations lead to destruction of the protein kinase fold, and how ubiquitin can be phosphorylated. Structures of proteins are important to enable drug discovery efforts; in case of PINK1, small molecule activators may be clinically useful for the treatment of Parkinson’s disease.
The Lister Institute is very pleased to play a role in funding such high profile research – our goal is to help support scientists who are really driving the biomedical sector forward in the UK and Republic of Ireland, and David’s work is a great example of this.
Find out more about how our research fellowships are helping scientists like David Komander to push the boundaries of our understanding.



