
Former Lister Fellow and member of the Lister’s Scientific Advisory Group (SAC) Magdalena Zernicka-Goetz has grown a model mouse embryo from stem cells.
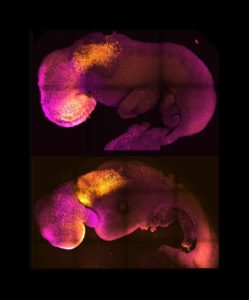
Magdalena and her colleagues combined embryonic stem cells and two other types of stem cells from mice. By inducing the expression of a particular set of genes and establishing a unique environment for their interactions, the researchers were able to get the stem cells to interact. The stem cells self-organised into structures that progressed through the successive developmental stages until they had beating hearts and the foundations of the brain. The resulting structure also contained a yolk sac where the embryo develops and gets nutrients from in its first weeks.
This is a further point in development than has been achieved in any other stem cell-derived model.
“It’s just unbelievable that we’ve got this far. This has been the dream of our community for years, and a major focus of our work for a decade, and finally we’ve done it.”
The researchers found that the extraembryonic cells signal to embryonic cells by chemical signals but also mechanistically, or through touch, guiding the embryo’s development.
“This period of human life is so mysterious, so to be able to see how it happens in a dish – to have access to these individual stem cells, to understand why so many pregnancies fail and how we might be able to prevent that from happening – is quite special,” said Magdalena. “We looked at the dialogue that has to happen between the different types of stem cell at that time – we’ve shown how it occurs and how it can go wrong.”
The resulting “embryoids” mirror a natural mouse embryo up to 8.5 days after fertilisation.
With further refinement, the technique could improve understanding of the earliest stages of organ development, and why some pregnancies fail. It could also be used to better understand early stages of development and study mechanisms behind disease, avoiding the need to use animal models.
The research was published in Nature on 25 August.
Magdalena is Professor of Mammalian Development and Stem Cell Biology at the University of Cambridge and Bren Professor of Biology and Biological Engineering at the California Institute of Technology. You can find out more about her research here.



